By the end of the unit, the students will be able to:
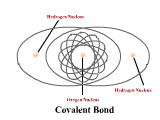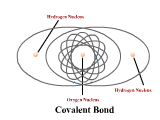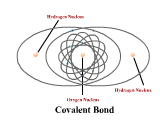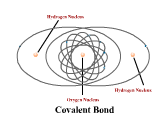
1 - Explain how atoms combine to form compounds through both ionic and covalent bonding. Predict chemical formulas based on the number of valence electrons. (4.1)
2 - Use electronegativity to explain the difference between polar and nonpolar covalent bonds. (4.3)
3 - Explain why chemical bonds occur.
4 - Define the octet rule and explain its application in chemical bonds.
5 - Draw Lewis dot structures for simple molecules and ionic compounds. (4.2)
6 - Describe the process of metallic bond formation.
7 - Use valence-shell electron-pair repulsion theory (VSEPR) to predict the molecular geometry (linear, bent, triangular planar, triangular pyramidal and tetrahedral) of simple molecules. (4.4)
8 - Identify how hydrogen bonding in water affects a variety of physical, chemical, and biological phenomena (e.g., surface tension, capillary action, density, boiling point). (4.5)